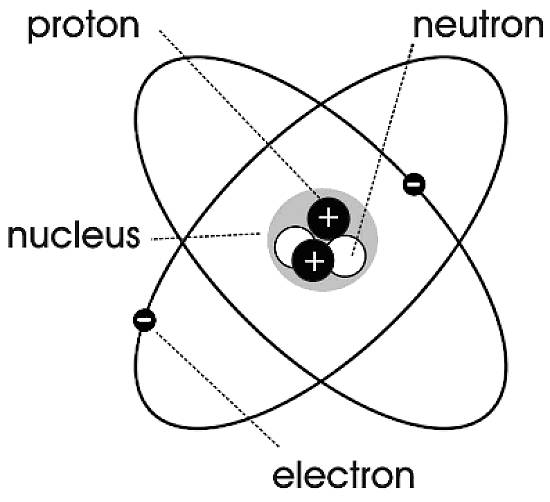
 | John Dalton thought atoms were indivisible, but investigators in the late 1800's proved otherwise. As advances in science became more prominent, it allowed for deeper exploration of matter and it became clear that atoms are composed of smaller subatomic particles. The number and arrangement of these particles in an atom determine the atoms chemical properties. All atoms have two regions: The Nucleus: a very small region towards the center of an atom, and an outer region. There are also three subatomic particles that make up an atom: Protons: positively charged particles located in the nucleus; Neutrons: neutral charged particles located inside the nucleus; and Electrons: negatively charged particles located outside the nucleus. (pg.72) |
Discovery of the Electron
In the late 1800s, many experiments were performed where an electric current passed through various gases at low pressure. These experiments were conducted in in glass tubes called cathode-ray tubes. Investigators noticed that when a current passed through the tube, the surface oposite of the cathode glowed. They came to a conclusion that the glow was from a stream of particles, which was later called a cathode ray. This ray travled from the cathode to the anode. (pg. 72)
"Experiments devised to test this revealed the following observations: 1.) Cathode rays were deflected by a magnetic field in the same manner as a wire carrying electric current, which was known as a negative charge. (see figure) 2.) The rays were deflected away from negatively charged objects." (pg. 72-73) These observations led to the hypothesis that the particles that made up cathode rays are negatively charged and this hypothesis was strongly supported by a series of experiments done by Joseph John Thomson in 1897. In one of his investigations, he was able to measure the ratio of the charge of the cathode-ray particles to their mass. He found that this ratio was the same regardless of the metal used to make the cathode or the nature of the gas inside the tube. He concluded that the rays are composed of identical negatively charged particles, which were later named electrons. (pg 73) | 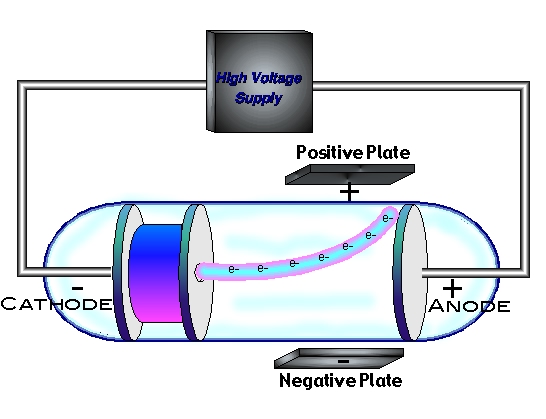 |
 | Charge and Mass of the Electrons
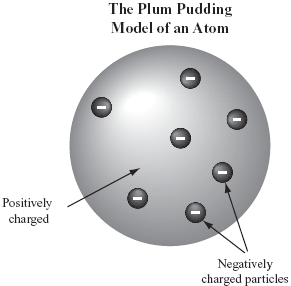
In 1909, Robert A. Millikan measured the charge of the electron by a series of experiments. Scientists have since then used this information and the charge-to-mass ratio of the electron to find the mass of the electron, which is about one two-thousandth the mass of the simplest type of hydrogen atom; the smallest atom known. A more accurate measurement of the mass is 9.109x10^-31 kg. (pg 73) Based on what was learned about electrons, two other inferences were made about atomic structure: 1.) Because atoms are electrically neutral, they must contain a positive charge to balance the negative electrons. 2.) Because electrons have so much less mass than atoms, atoms must contain other particles that account for most of their mass. (pg 73 Thomson also proposed a model for the atom called the plum-pudding model, which he believed that the negative electrons were spread evenly throughout the positive charge of the rest of the atom. (see figure) (pg 73) |
Discovery of the Atomic Nucleus In 1911, Ernest Rutherford and his associates Hans Geiger and Ernest Marsden, shot fast-moving alpha particles, which are positively charged particles that have a mass 4 times that of a hydrogen atom, at a thin piece of gold foil. Both of his assistants thought that the mass and charge were the same all the way through the gold foil. They expected the alpha particles to go through, which most did, but some were deflected. (pg 74) After Rutherford thought for a few months, he had an explanations why they were deflected. He thought that there must have been something powerful with in the atom. He also figured out that this force must be extremely same because a majority of the particles moved through and only a few were deflected. He called this small force the nucleus. He also discovered that the volume of the nucleus was much smaller compared to the total volume of an atom. (pg 74) | 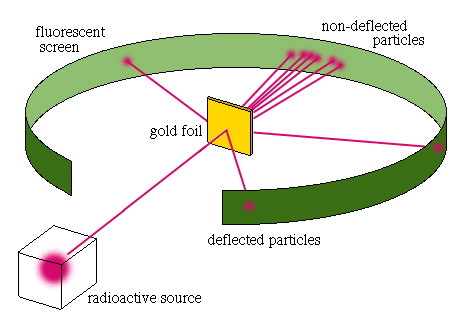 |
Composition of the Atomic Nucleus
All atomic nuclei are made of two kinds of particles, protons and neutrons. A proton has a positive charge that is equal to the negative charge of an electron. Atoms are electrically neutral because they contain equal numbers of protons and electrons. A neutron has a neutral charge. A proton has a mass of 1.673x10^-23 kg, which is much large than that of an electron. The mass of a neutron is 1.675x10^-23 kg, which is slightly larger that a protons mass. The nuclei of different elements differ in their number of protons, which means the number of protons gives an atom its identity. (pg 75)
Usually, particles that have the same electrical charge repel one another. When to protons are extremely close to each other, there is a strong attraction between them. "These short-range proton-neutron, proton-proton, and neutron-neutron forces hold the nuclear particle together are called Nuclear forces." (pg 76)
Works cited:
Alphy, Emily. "The Geiger–Marsden Experiment." Physics World RSS. Physics World, 2011. Web. 03 Oct. 2013.
Davis, R., Frey, R., Sarquis, M., & Sarquis, J. (2009). Modern chemistry. Orlando, FL: Holt, Rinehart, and Winsten
M., Eric. "Collaborative Chemistry." : JJ Thomson's Experiments with Cathode Ray Tubes. Blogger, 6 Oct. 2010. Web. 03 Oct. 2013.
"Massachusetts Comprehensive Assessment System." 2007 MCAS Sample Student Work. Massachusetts of Elementry & Seconday Education, 30 June 2013. Web. 03 Oct. 2013.